The National Haemophilia Database
The NHD collects, holds, processes and analyses confidential data for both research (non-invasive / non-interventional ) and non-research purposes (direct care, planning and commissioning). This information is kept in the National Haemophilia Database, which is located in Manchester. You need to be on the secure NHS network to access the database.
The National Haemophilia Database is being redeveloped to make it not only a fit for purpose database for the modern digital age but will enable us to develop new services and provisions for all key stakeholders that will take us to 2030 and beyond. Roche, CSL Behring, Pfizer, Sobi and Takeda has provided a financial grant to support the redevelopment of the National Haemophilia Database.
2021 Information Governance Assessment of the National Haemophilia Database – We are compliant!
The Information Governance Team at Manchester University NHS Foundation Trust (MFT) conducted an inspection of the NHD office and additionally, all our information governance arrangements including our polices, processes and procedures to assess our compliance with the Data Protection Act 2018 (DPA); General Data Protection Regulation (2016); Caldicott Principles and prevailing NHS Information Governance, guidance, legislation, and good practice as appropriate.
The inspection concluded on 30 June 2021, where MFT provided a comprehensive report on our arrangements which covered the following inspected areas: the NHD’s purpose, governance, staffing, suppliers, transparency, subject access requests, data quality and technical controls.
MFT’s report provides assurance that all our arrangements are compliant with all the relevant information governance regulations, Caldicott Principles and NHS legislation, guidance, and best practice. Their summary of the outcome can be found below:
MFT Summary:
“The Information Governance framework surrounding the National Haemophilia Database demonstrates a high level of process maturity. The governance actively engages data subjects in decision making about how their information is used and the management team were able to evidence this. There is appropriate separation of concerns in the design of the organisational structures with the intention of protecting personal information. As a small organisation they have engaged with suppliers who have the necessary credentials to provide a secure IT solution. The organisation is about to embark on a major upgrade of its technical platform. A further audit will be appropriate once this work has been completed.”
Patient Information changes
On September 2020, we were successful in applying to the Health Research Authority Confidentiality Advisory Group for Section 251 support in England and Wales. This in effect permits the NHD to collect, hold, process, analyse confidential data for both research and non-research purposes and to transfer confidential data between parties without there being a breach of our common law duty of confidentiality. We applied for this for a number of reasons:
- If any patients recorded in the database dies, we can be notified of their deaths and the details such as the date and cause, without obtaining informed consent. It is a NHS Digital requirement that we have this support when applying for mortality data.
- With the global Covid-19 pandemic consultations are being conducted virtually, and where appropriate, patients receive product at home to enable them to self-medicate. This has reduced the amount of opportunities for consent to take place.
However, as this support removed the requirement to seek a patient’s consent, the new patient information that has been drafted provides detail on the persons data rights, and presents them with the clear opportunity to opt out of their data being used for research. This would usually take place at the Haemophilia Centre during consultation, but if the person wants to think about it, or change their mind, they can contact us direct and we will process their rights to opt out. For further information on Section 251, please follow the HRA link here: https://s3.eu-west-2.amazonaws.com/www.hra.nhs.uk/media/documents/cag-frequently-asked-questions-1.pdf
In September 2021 the Scottish Public Benefit and Privacy Panel for Health and Social Care (PBPP) gave approval for Haemophilia Centres in Scotland to share data with the NHD to enable it to collect, store and process confidential data for research and non-research purposes, without written consent from the individuals concerned. This agreement from PBPP recognises the practical difficulties in obtaining written informed consent, for example, from people lost to follow up or deceased.
The consent process will remain in Northern Ireland until further notice. We have drafted new patient information which have been consulted on with the Health Research Authority, NHS Digital, NHS Scotland, Northern Ireland Privacy Advisory Committee, HSC Business Services Organisation (NI), UKHCDO members and Patient Representatives. To ensure there is no confusion we have added the relevant patient information by each region.
England and Wales
Broadcast – this is a ‘one pager’ to meet NHS digital, HRA (CAG) requirements and patient representative requirements. It highlights approval of section 251 support, the exchange of identifiers for mortality purposes and patients’ rights to opt out for research purposes. It also includes the National Data Opt-Out Service. The purpose of this is to make this available in laminated A3 format in centres, but can also be used patient representative organisations websites. NHD One Page Broadcast v5.0 England and Wales.pdf
NHD Information Leaflet (your questions answered) – This is a redrafted version of the leaflet that can be printed and folded and which can be used in Haemophilia centres. NHD Information Leaflet 2021 v5.0 England and Wales.pdf
Data Opt-out Form – For England and Wales, with approval of section 251 support, we have moved away from the consent model for research purposes. We need to make sure we are explicit in providing a process for patients to opt-out of their data being used for research purposes. The opt our form will be made available to all Haemophilia centres. Patients will also be able to contact the NHD direct. NHD Patient Research Opt-Out Form v.3.0 England and Wales.pdf
Scotland
The Scottish Public Benefit and Privacy Panel for Health and Social Care (PBPP) gave approval in September 2021 for Haemophilia Centres in Scotland to share data with the NHD to enable it to collect, store and process confidential data for research and non-research purposes, without written consent from the individuals concerned. This agreement from PBPP recognises the practical difficulties in obtaining written informed consent, for example, from people lost to follow up or deceased.
NHD Information Leaflet (your questions answered) – This is a redrafted version of the leaflet that is being used in Scotland that can be printed and folded and which can be used in Haemophilia centres. Scotland Patient Information Leaflet 2021 v3.0.pdf
Data Opt-out Form – For Scotland, with approval of the Scottish Public Benefit and Privacy Panel for Health and Social Care support, we have moved away from the consent model for research purposes. We need to make sure we are explicit in providing a process for patients to opt-out of their data being used for research purposes. The opt-out form will be made available to all Haemophilia centres. Patients will also be able to contact the NHD directly. NHD Patient Research Opt-Out form v3.0 Scotland.pdf
Northern Ireland
NHD Information Leaflet (your questions answered) – This is a redrafted version of the leaflet for Northern Ireland that can be printed and folded and which can be used in Haemophilia centres. NI Patient Information Leaflet 2021 v3.0.pdf
Patient Consent form – Haemophilia Centres staff should use the NHD website (https://nww.mdsas.nhs.uk/nhd) to access patient consent forms. This requires access to the secure NHS network and a valid user account.
Access to National Haemophilia Database health records application form
Please download and complete the Subject Access Request form to request you Health Records from the National Haemophilia Database. Included in this document are all the necessary details of how to do this. If you are a patient representative please download the form specifically for a Patient Representative.
National Haemophilia Database Dataset
This dataset is consulted on with key stake holders for direct patient care, planning, commissioning, and research purposes through the Data Management Working Party
This includes lists of additional data points collected if an adverse event, such as a new factor VIII inhibitor, is reported to the database.
This list bears no relationship to data collected in previous years and should not be taken as a guide to the information collected in the past. Data collected in the earlier years of the database was very limited but has widened gradually with the passage of time.
Download the NHD Dataset 2020 –  650kb
650kb
Obtaining Data from the National Haemophilia Database
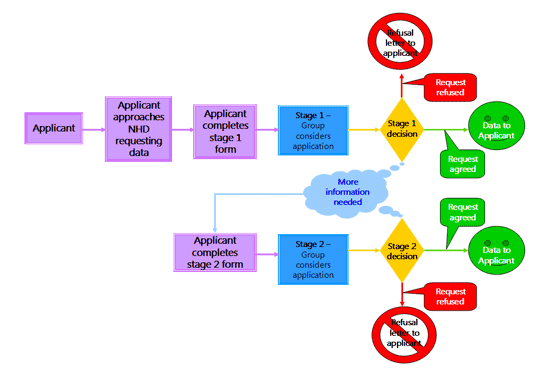
To request data from the the National Haemophilia Database, complete the form.
The procedure for reviewing an application can be viewed below.
Please read all of the questions prior to completing the form, they can be found here.
Request for data from the NHD flow
NHD Contact Details
| Administrators: |
|---|
| Mr Alex Godsall UKHCDO Suite 1, 2nd Floor of Anchorage One Anchorage Quay Salford Quays M50 3YJ |
| Tel : 0161 850 8102 Email: support@ukhcdo.org |